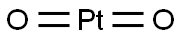Platinum dioxide:Application and Chemical Studies
General Description
Platinum dioxide, also known as Adams catalyst, as a noble metal oxide catalyst, has a wide range of applications in hydrogenation, hydrogenolysis, dehydrogenation and oxidation reactions.Due to its excellent catalytic performance, high activity, high selectivity, long service life and recyclability, noble metal oxide catalysts play an extremely important role in the fields of petroleum, chemical industry, medicine, pesticide, and energy.[1]A group commonly used for hydrogenation or hydrogenolysis of an olefinic bond, a hydroxyl group, an aldehyde group, an imido group, an aromatic nitro group, and an aromatic ring.[2]Platinum oxide, a safe-to-handle-and-store, eco-friendly and typically solid metallic oxide, has been widely utilised as a catalyst in catalytic hydrogenation.[3]Platinum dioxide properties can be optimized by plasma oxidation of sputtered PtOx based on previous researches.[4]

Figure 1 Powder of the Platinum dioxide
Preparation
The current synthesis method about platinum dioxide is generally prepared by melting chloroplatinic acid or ammonium chloroplatinate and sodium nitrate at 500°C. In order to ensure the complete reaction, the input of sodium nitrate is greatly excessive, and the prepared platinum dioxide has large particles, etc. The distribution specific surface area of the amount of platinum dioxide in the catalyst is relatively small, and the area in contact with the gas is relatively small, and there is a phenomenon of waste.In order to overcome the defects in the prior art, a preparation method of platinum dioxide catalyst, comprising the following methods:
The great preparation method for the platinum dioxide includes the following steps:Step1:taking 100g of platinum tablets, 300mL of nitric acid with a mass fraction of 65%, and 400mL of hydrochloric acid with a mass fraction of 36%, mix the platinum tablets and the two acids and heat them at 90°C. After the platinum tablets are completely dissolved, they are concentrated to obtain 250mL of mixed solution. Then add 250mL of water to obtain an acid solution of platinum;
Step 2:the acid solution of platinum was mixed with 10g of silica with a particle size of 18 μm, stirred at 300rpm for 10min, and after stirring, ultrasonicated at 70KHz and 20°C for 45min to obtain a dispersion solution;
Step 3:mix the dispersion solution with 700g of sodium nitrate, keep the rotation speed at 75 rpm, heat up to 380°C at a heating rate of 8 °C/min under stirring for 4 hours to complete the first reaction, and then heat up to 545°C at a heating rate of 12°C/min. 4h completes the second reaction, and after the second reaction finishes, natural cooling obtains black product;
Step 4:the black product was fully dispersed in water, heated at 70°C for 50min to remove the salt in the product, then suction filtered at -0.1Mpa to obtain powder, and the powder was washed once with 200mL of water for a total of five times; The platinum dioxide catalyst was obtained by drying at 120°C for 10 h. In this example, 130.71g of catalyst was obtained, the detected platinum content was 76.47%, the yield was 99.95%, and the detected specific surface area was 129m2/g.The method provided by the invention has high efficiency, the yield of platinum dioxide catalyst can reach 99.99%, and the specific surface area detection can reach 141m2/g, which is a catalyst with excellent performance.[1]
Application
Using platinum dioxide or platinum dioxide/nickel oxide (NiO) as the catalyst, A practical, facile and highly efficient transesterification reaction under essentially neutral conditions can be achieved according to the previous literature.
Some researches reveal that platinum oxide is a convenient, highly efficient and environmentally friendly catalyst in the transesterification reaction. The transesterification reaction mediated by platinum oxide under mild and neutral conditions effectively avoids the corrosivity of acid and alkali, the high toxicity of organo-metallic catalysts and the generation of side reactions. Furthermore, the products are easy to separate.Meanwhile, the study also shows that a mixture of nickel oxide (NiO)/platinum dioxide results in the same catalytic activity to the reaction as that of platinum dioxide. In the presence of NiO, the catalytic amount of platinum dioxide is sufficient to mediate the reaction effectively, and the results are similar to those of platinum dioxide.[3]
Reference
[1]Xie Z,Liu B,et al.Platinum dioxide catalyst with excellent performance and its preparation method[P]CN114029051. 2022.
[2]Xu H.Method for preparing platinum dioxide particle with uniform particle size distribution, and its application as multiphase Adams catalyst[P].CN109174083.2019.
[3]Teng B, Shi J, Yao C. Facile, highly efficient and environmentally friendly transesterification mediated by platinum dioxide and nickel oxide under essentially neutral conditions[J]. Green Chemistry, 2018, 20(11):2465-2471.
[4]Basu N, Sterin N S, Mamidala S R, et al. Optimization of Platinum dioxide properties by plasma oxidation of sputtered PtOx[J]. Materialia, 2019,8:100477.
You may like
Related articles And Qustion
See also
Lastest Price from Platinum dioxide manufacturers

US $30.00-10.00/g2025-11-03
- CAS:
- 1314-15-4
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 300tons

US $1.00/KG2025-06-27
- CAS:
- 1314-15-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt